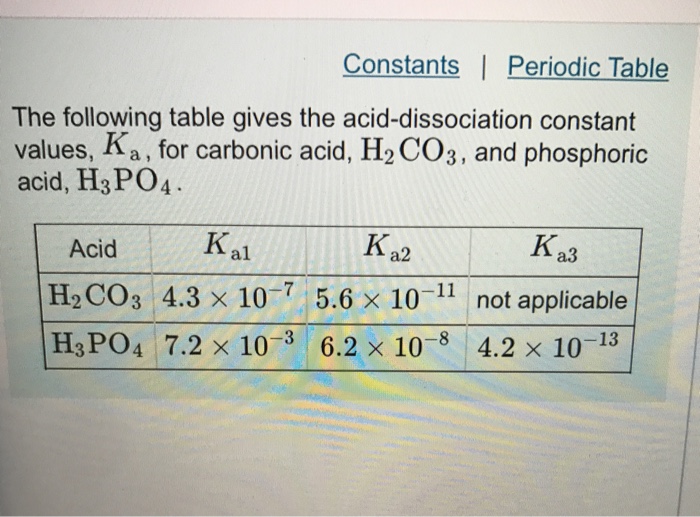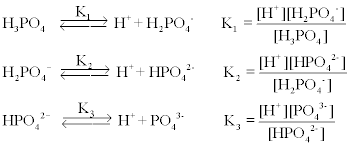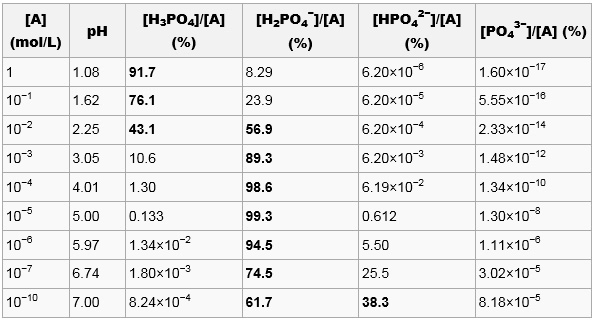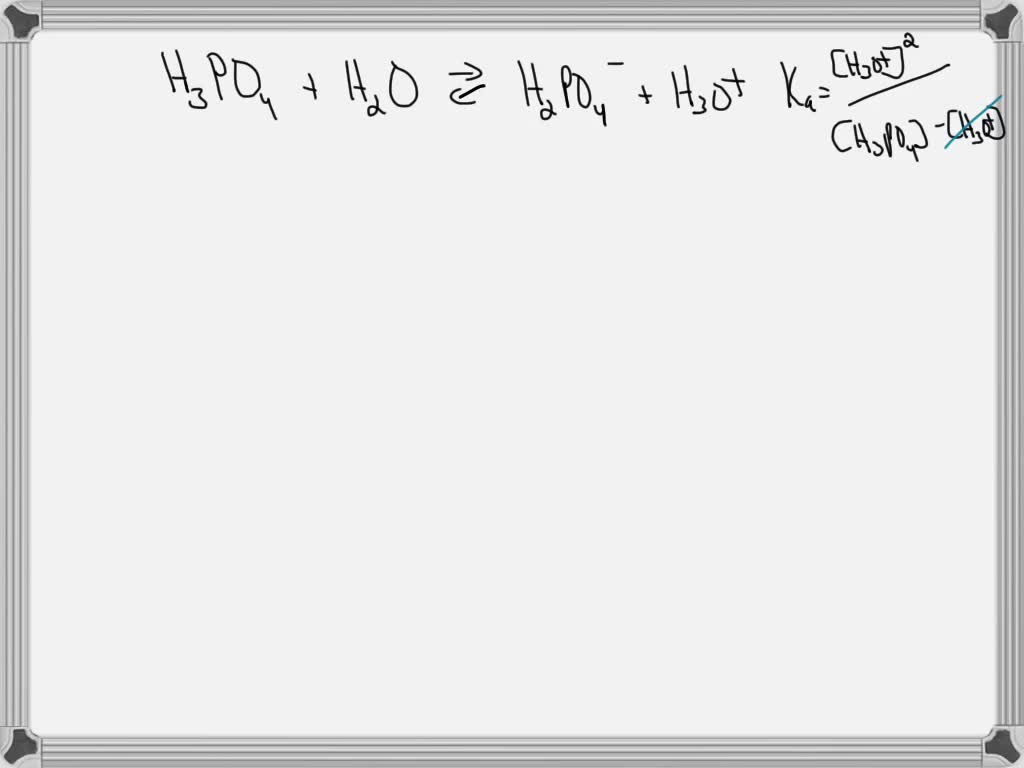
SOLVED: The acid-dissociation constants of phosphoric acid, H3PO4, are Ka1 = 7.5 × 10-3 , Ka2 = 6.2 × 10-8 and Ka3 = 4.2 × 10-14 at 25.0°C. What is the pH of a 7.40 M aqueous solution of phosphoric acid?.
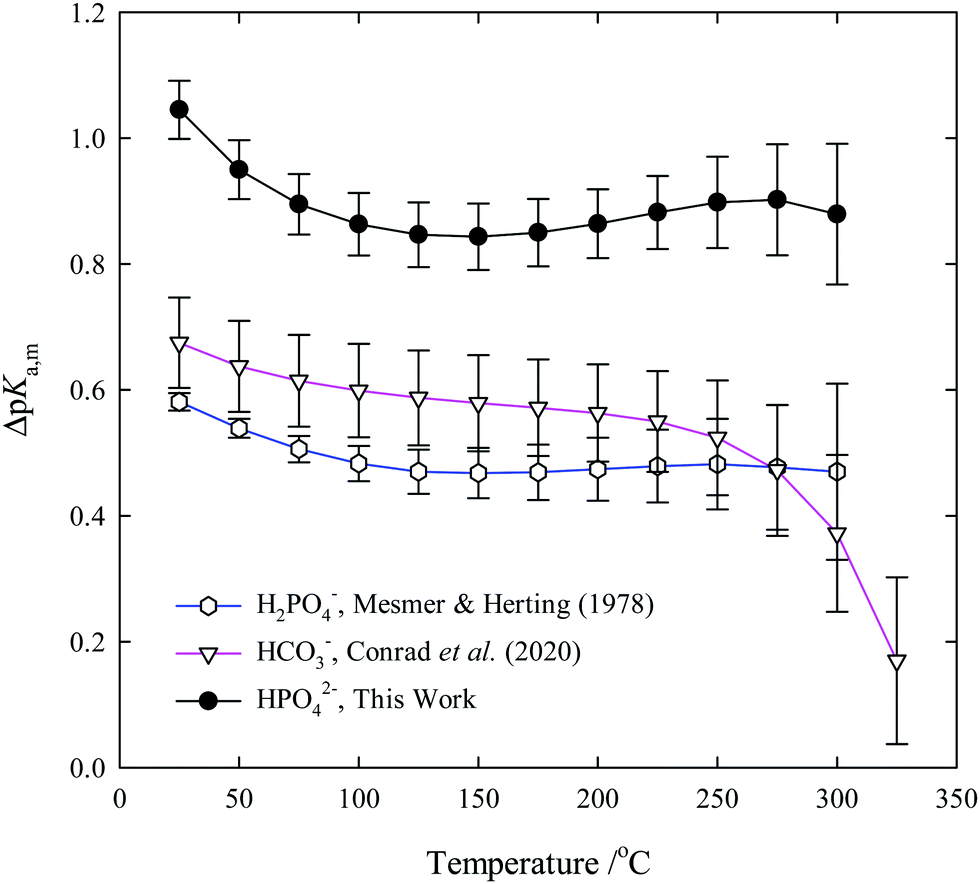
Third dissociation constant of phosphoric acid in H 2 O and D 2 O from 75 to 300 °C at p = 20.4 MPa using Raman spectroscopy and a titanium-sapphire f ... -

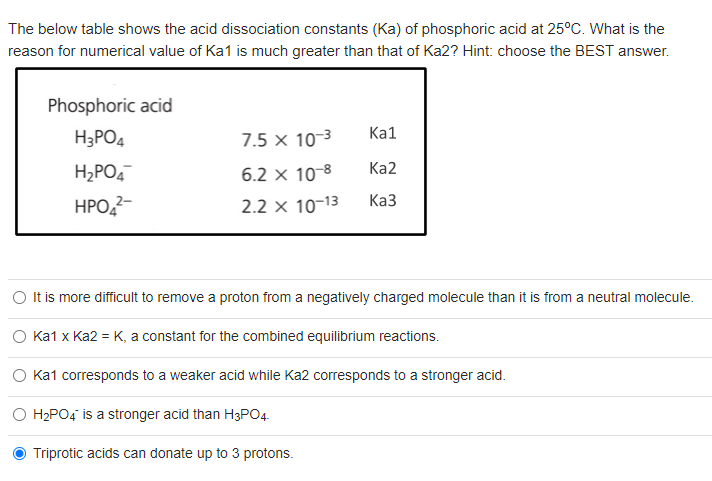
Phosphoric acid is a triprotic acid with the follow Ka values: Ka1 = 7.2x10−3, Ka2 = 6.3x10−8, Ka3 = - Brainly.com

Chemistry Laboratory: Neutralization of a polyprotic acid with a strong base (Key words: Phosphoric acid)
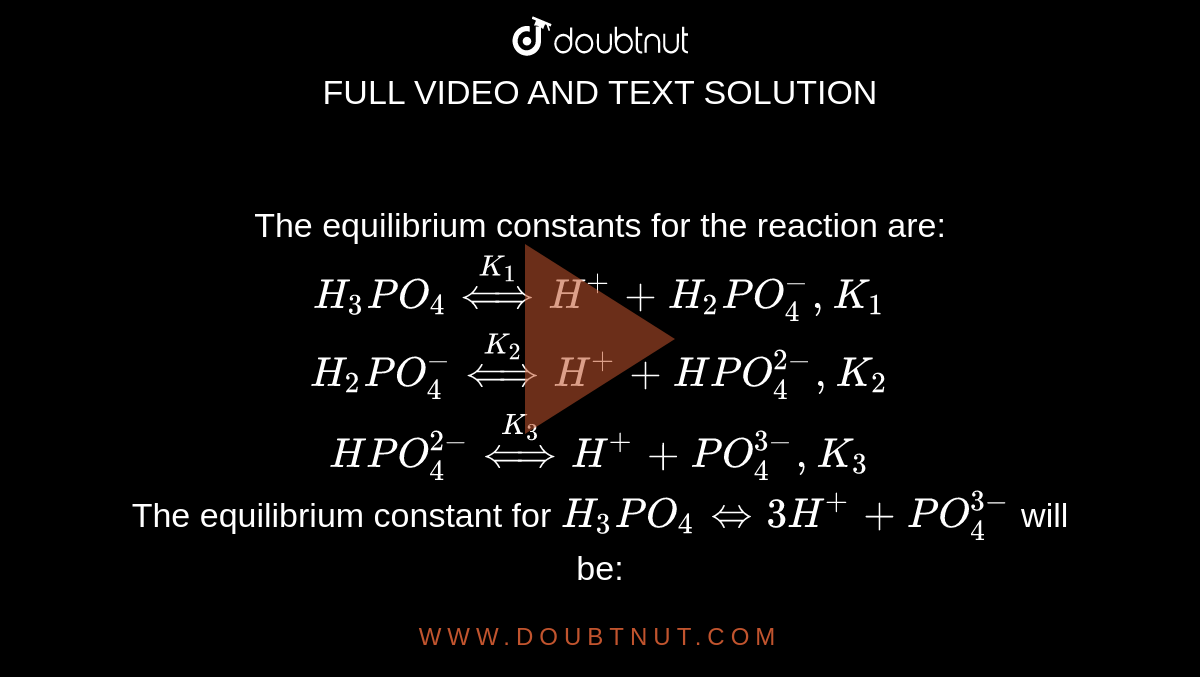
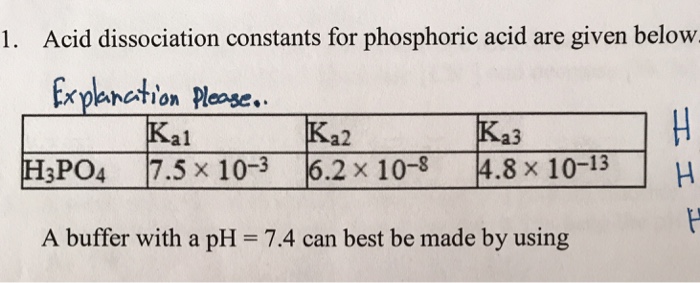
For a polyprotic acid, H(3)PO(4) its three dissociation constanst K(1),K(2) and K(3) are in the order

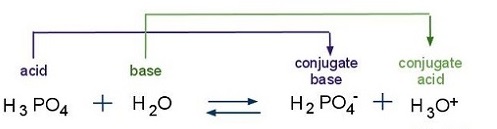
First ionization of phosphoric acid is: H3PO4 H3PO4^- + H^+ pKa1 = 2.21 The dissociation constant of conjugate base of H3PO4 will be:

Acid dissociation constants and cytotoxicity test of a series of omega-aminoalkyl phosphates - ScienceDirect
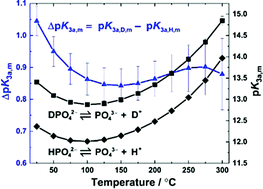
Third dissociation constant of phosphoric acid in H2O and D2O from 75 to 300 °C at p = 20.4 MPa using Raman spectroscopy and a titanium-sapphire flow cell - Physical Chemistry Chemical Physics (RSC Publishing)
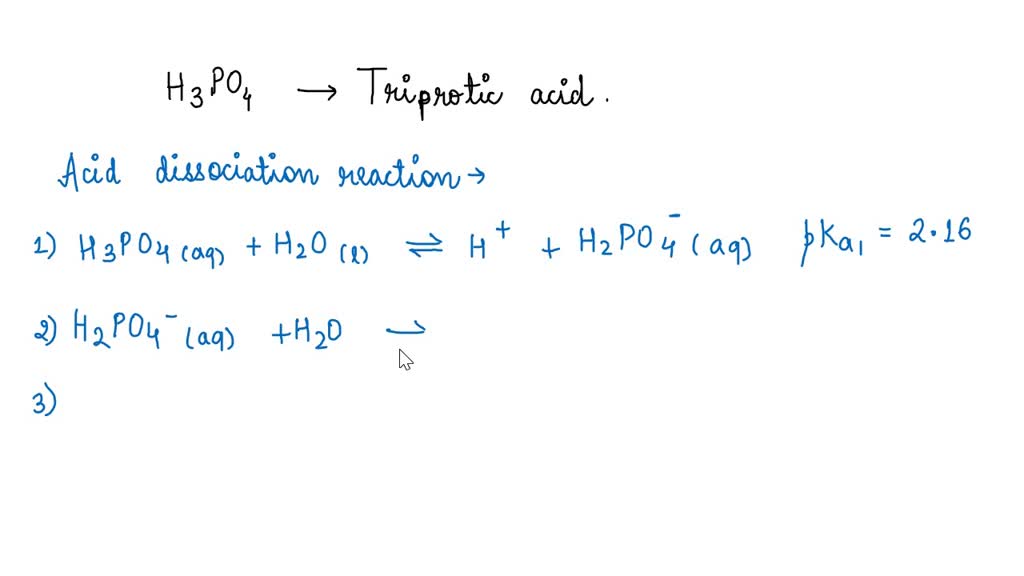
SOLVED: Phosphoric acid, H3PO4 is a triprotic acid. pKa1= 2.16 pKa2=7.21 pKa3=12.32 Write three separate acid dissociation reactions for the three acidic protons. Make sure to indicate which Ka and Kb value