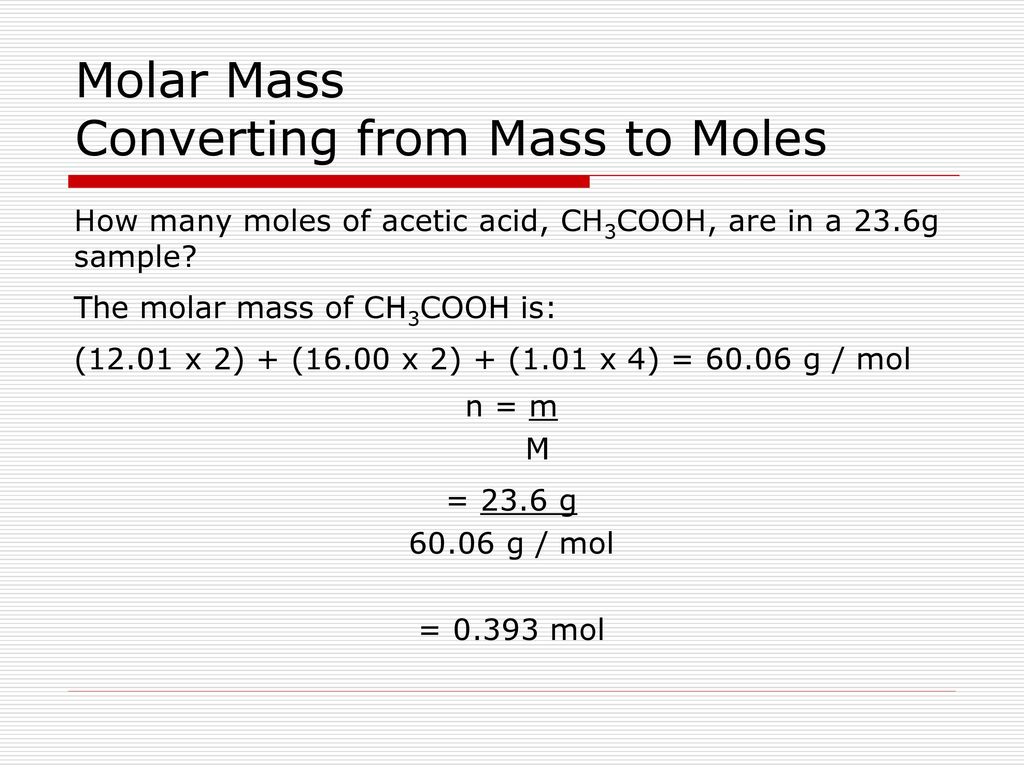
SOLVED: Moles of acetic acid in 10 mL vinegar solution (0.8361*10)/1000 0.0084 Molar mass of acetic acid 60 g/mol So, amount of acetic acid present in 10 mL vinegar solution 0084) 0.504
![SOLVED: MgPO3 MgPOz The empirical formula of acetic acid is CH2O and its molar mass 60.0 determine molecular formula acetic acid (abi; 1] C = 12.0, H = 1.01, 0 = 16 MO CzH40z CzH6O3 C4Hs04 CHz0 SOLVED: MgPO3 MgPOz The empirical formula of acetic acid is CH2O and its molar mass 60.0 determine molecular formula acetic acid (abi; 1] C = 12.0, H = 1.01, 0 = 16 MO CzH40z CzH6O3 C4Hs04 CHz0](https://cdn.numerade.com/ask_images/90f47f1c11324fe987926927a56d1388.jpg)
SOLVED: MgPO3 MgPOz The empirical formula of acetic acid is CH2O and its molar mass 60.0 determine molecular formula acetic acid (abi; 1] C = 12.0, H = 1.01, 0 = 16 MO CzH40z CzH6O3 C4Hs04 CHz0

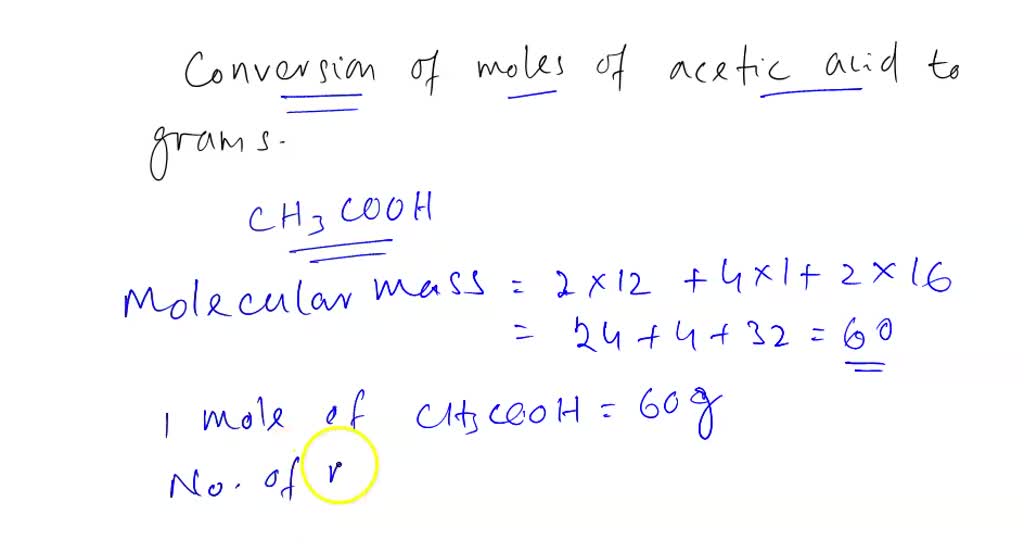
Calculate the molecular mass of ethanoic acid, `CH_(3)COOH`. (Atomic masses : `C = 12 u , H = 1 u , - YouTube

Statement-1 : The observed molar mass of acetic acid in benzene is more than the nomal molar mass of - YouTube

The experimental molecular weight of acetic acid is double the theoretical molecular weight of acetic acid. Why? - Quora

Calculate the molecular mass of ethanoic acid, CH3COOH. (Atomic masses C = 12 u ; H = 1 u ; O = 16 u) - Brainly.in

Plan for Fri, 26 Sept 08 Diagnostic Quiz returned –Average = /- 18.1% Lecture –Naming acids and their anions (2.8) –Counting by weighing (3.1) –More. - ppt download
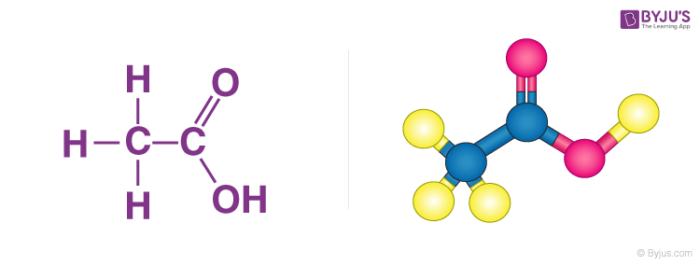
Acetic Acid (CH3COOH)- Structure, Properties, Preparation, Physical, Chemical properties, Uses and FAQs of Acetic acid

205 molarity acetic acid solution is having density 102g cm3 find molality of the solution if molecular mass of the acetic acid is 60g 7s86fj77 -Chemistry - TopperLearning.com

Determination of the molar mass of acetic acid in benzene using freezing point depression is - YouTube

Molar Mass. Molecular Mass The molecular mass of a substance is the mass in atomic mass units (amu) of all the atoms in a given molecule. It is more commonly. - ppt

Calculate the mass of ascorbic acid (C6H8O6) to be dissolved in 75 g of acetic acid to lower its melting point by 1.5^0C . (Kf for acetic acid is 3.9 K kg mol^-1 ).

Moles to Mass. Calculating Molar Mass Calculate the mass of 1 mole of Carbon Dioxide (CO 2 ) M CO 2 = g/mol + 2(16 g/mol) = 44 g/mol. - ppt download

Density of 2.03 Maqueous solution of acetic acid is 1.017 g /ml .molecular mass of acetic acid is 60. - Brainly.in