Solution For: Write two equations for the neutralization of nitric acid, HNO, with magnesium hydroxide, - Brainly.com

Mg(OH)2+HNO3=Mg(NO3)2+H2O Balanced Equation||Magnesium Hydroxide+Nitric acid =Magnesium Nitrate+Water - YouTube
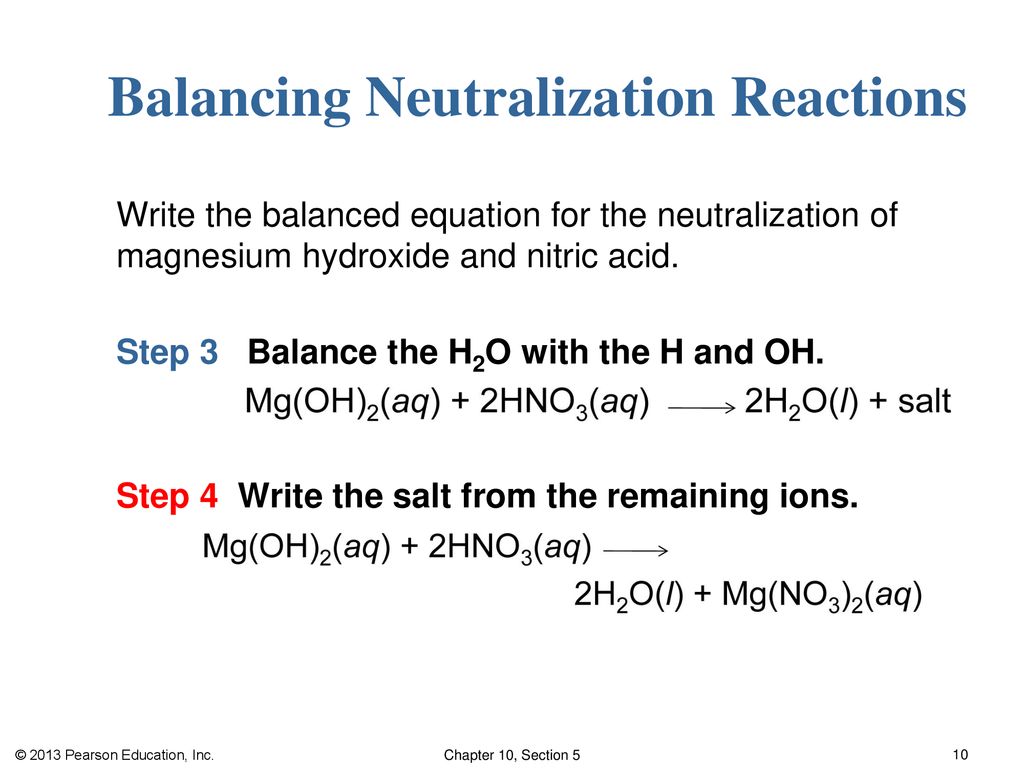
Acids and Metals Acids react with certain metals to produce hydrogen gas and the metal salt. metal acid metal salt. - ppt download
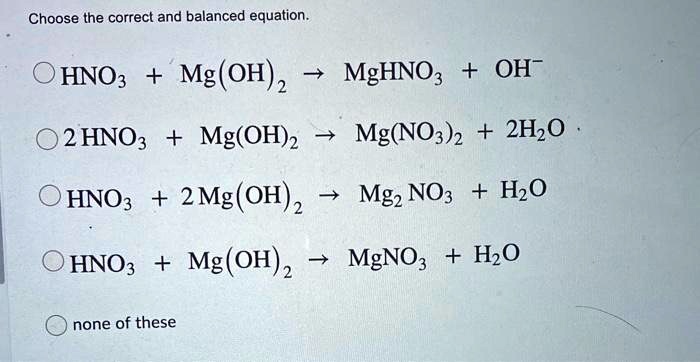
SOLVED: Choose the correct and balanced equation. HNOz MgC (OH) 2 MgHNO: OH- 2 HNO3 Mg(OH)2 Mg(NO3) + 2Hz0 HNO3 + 2 Mg(OH) 2 Mgz NO3 + HzO HNO3 Mg(OH) 2 MgNOz +

Write the balanced chemical equation for the following and identify the type of reaction in each case.a) Calcium hydroxide (aq) + Nitric acid (aq)→Water (l) + Calcium nitrate (aq) b) Magnesium (s) +
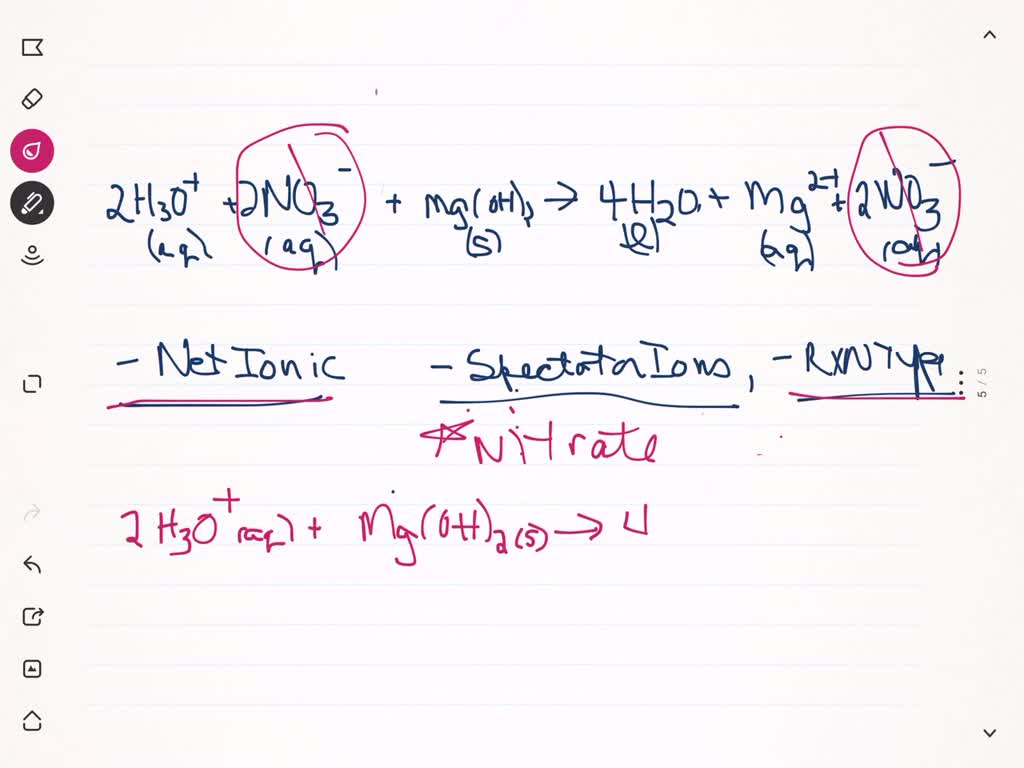
SOLVED:Write the net ionic equation and identify the spectator ion or ions in the reaction of nitric acid and magnesium hydroxide. What type of reaction is this? 2 H3O^+(aq)+2 NO3^-(aq)+Mg(OH)2(s) → 4
What is the balanced chemical equation for the reaction that occurs between nitric acid (HNO3) and aluminum hydroxide? - Quora

4. Write the balanced chemical equation for the following and indentify the type of reaction 17. each case. - Brainly.in










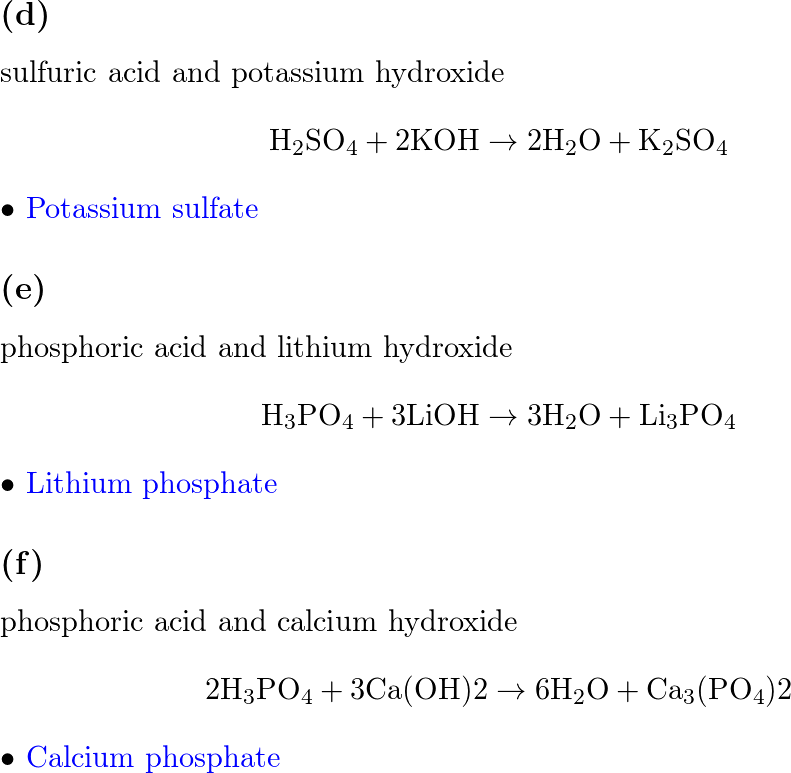
.PNG)